Researchers led by a team at the Exploratory Research Center on Life and Living Systems (ExCELLS) and the National Institute for Physiological Sciences (NIPS) have developed a new method for in vivo brain imaging, enabling large-scale and long-term observation of neuronal structures and activities in awake mice. Their nanosheet incorporated into light-curable resin (NIRE) method uses fluoropolymer nanosheets covered with light-curable resin to create larger cranial windows.
The new method represents what the team claims is a significant achievement in the field of neuroimaging and will allow researchers to investigate neural processes that were previously difficult or impossible to observe. The NIRE method provides a platform for investigating neuroplastic changes at various levels over extended periods in animals that are awake and engaged in various behaviors, which presents new opportunities to enhance understanding of the brain’s complexity and function.
The ability to create large cranial windows with prolonged transparency and fewer motion artifacts should allow for large-scale, long-term, and multi-scale in vivo brain imaging, they suggested. “The method holds promise for unraveling the mysteries of neural processes associated with growth and development, learning, and neurological disorders,” said senior author Tomomi Nemoto, PhD, at ExCELLS and NIPS. “Potential applications include investigations into neural population coding, neural circuit remodeling, and higher-order brain functions that depend on coordinated activity across widely distributed regions.”
Nemoto and colleagues reported on the NIRE method in Communications Biology, in a paper titled “Large-scale cranial window for in vivo mouse brain imaging utilizing fluoropolymer nanosheet and light-curable resin,” in which they concluded, “The NIRE method can facilitate in vivo large-scale analysis of heretofore inaccessible neural processes, such as the neuroplastic changes associated with maturation, learning and neural pathogenesis.”
The human brain has billions of neurons. Working together, they enable higher-order brain functions such as cognition and complex behaviors. To study these higher-order brain functions scientists need to understand how neural activity is coordinated across various brain regions. Although techniques such as functional magnetic resonance imaging (fMRI) are able to provide insights into brain activity, they can show only so much information for a given time and area. “ … while such methods enable the imaging of brain activity over broad regions, temporal and spatial resolution are limited,” the authors noted.
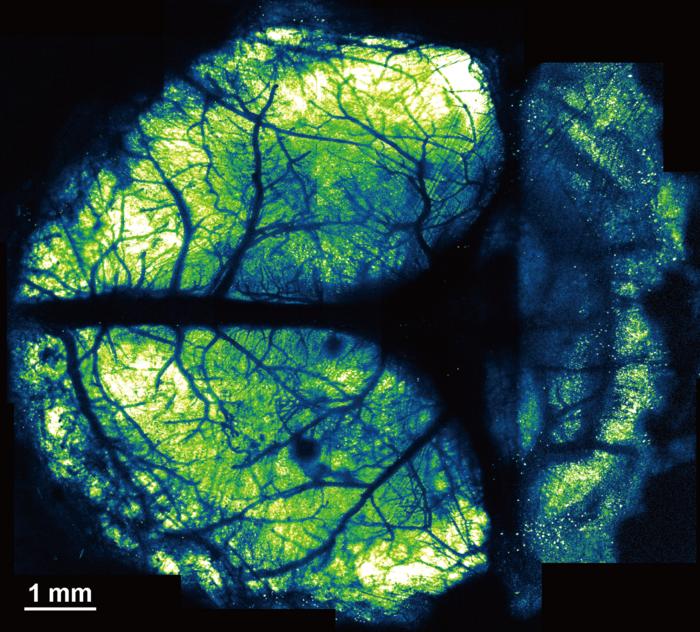
Two-photon microscopy, involving the use of cranial windows, is a powerful tool for producing high-resolution images but conventional cranial windows are small, making it difficult to study distant brain regions at the same time. A cranial window is typically created by removing a portion of the skull (craniectomy) and sealing the hole with a glass coverslip, the authors explained. “However, conventional cranial windows are usually small (2–4mm in diameter) to minimize bleeding, avoid damage to large blood vessels, and prevent mechanical stress on curved brain tissue caused by a flat glass coverslip.” And to date, methods have yet to be developed for creating larger cranial windows that are big enough to simultaneously image distant regions or the cerebral cortex, or cerebral cortex and cerebellum, the team pointed out.
The authors had previously proposed the use of polyethylene-oxide-coated CYTOP (PEO-CYTOP) nanosheets as a sealing material for creating larger cranial windows. “These nanosheets possess unique properties amenable to in vivo two-photon microscopy, including high adhesion strength, flexibility, and transparency.” For their newly reported study, the team developed the NIRE method, by which light-curable resin is used to fix the bioinert, transparent PEO-CYTOP nanosheet onto the brain surface. This creates a window that fits tightly onto the brain surface—even the highly curved surface of the cerebellum— and maintains its transparency for a long time with little mechanical stress, allowing researchers to observe multiple brain regions of living mice. The cranial windows generated allowed for high-resolution imaging with sub-micrometer resolution, making them suitable for observing the morphology and activity of fine neural structures.
Reporting on their tests in mice, the researchers demonstrated that the NIRE method could produce cranial windows conforming the curved cortical and cerebellar surfaces, without motion artifacts, in awake mice, and maintain transparency for more than five months. “In addition, the NIRE method successfully enabled the visualization of neural structures and intracellular Ca2+ concentration changes at various scales, from populations of over a thousand neurons to single spines, in living mouse brain,” they wrote.
“The NIRE method is superior to previous methods because it produces larger cranial windows than previously possible, extending from the parietal cortex to the cerebellum, utilizing the biocompatible nanosheet and the transparent light-curable resin that changes in form from liquid to solid,” said lead said first author Taiga Takahashi, PhD, at Tokyo University of Science and ExCELLS. “Additionally, we showed that the combination of PEO-CYTOP nanosheets and light-curable resin enabled the creation of stronger cranial windows with greater transparency for longer periods of time compared with our previous method. As a result, there were few motion artifacts, that is, distortions in the images caused by the movements of awake mice,” says Takahashi.
“Importantly, the NIRE method enables imaging to be performed for a longer period of more than 6 months with minimal impact on transparency. This should make it possible to conduct longer-term research on neuroplasticity at various levels—from the network level to the cellular level—as well as during maturation, learning, and neurodegeneration,” added corresponding author Nemoto. “The method holds promise for unraveling the mysteries of neural processes associated with growth and development, learning, and neurological disorders. Potential applications include investigations into neural population coding, neural circuit remodeling, and higher-order brain functions that depend on coordinated activity across widely distributed regions.”
Reporting in their published paper, the team commented that their method “… facilitates the creation of cranial window large enough to view the cerebral cortex, midbrain, and cerebellum simultaneous for neural network studies. Collectively, these properties may facilitate the elucidation of neural network activities and neuroplastic changes underlying higher brain functions as well as disease processes in model animals.”
They suggest that the method could also have wider reaching applications. “Since it can effectively prevent motion artifacts without causing mechanical stress and inflammation, the NIRE method can be used for long-term in vivo imaging of various other organs,” they suggested. However, the team pointed out, “… a scaffold device (comparable to those used in previous studies) would be required to realize in vivo imaging of organs that lack a natural scaffold to fix the sealant (unlike brain, which has skull) while using the NIRE method. Additionally, a protective device similar to that used here (for brain) may also be needed to protect the transparency of the light-curable resin window, which deteriorates by contact with the mouse body and debris.”